
IEC 60601: Medical Electrical Equipment Testing and Certification
Mark your active medical devices as safe and reliable. They must complete compliance evaluation, testing and device approval to the international standards of IEC 60601 before being placed on the market. Any oversight of key compliance standards can be costly to the manufacturer, especially in the areas of product redesign, compliance testing, device approval and certification turnaround time. Expand your market access! Certify your active medical products now!
IEC 60601 is a widely accepted benchmark for medical electrical equipment and compliance. It has become a requirement for the commercialisation of electrical medical equipment in many countries. Many companies view compliance with IEC 60601 as a requirement for most markets.
Your Benefits At A Glance
1. Upfront pricing and customized service packages tailored to your needs.
2. Save time and money- by ensuring your product is compliant to global and local regulations from your first prototype, thereby avoiding costly delays in redesign.
3. Benefit from global support- with engineers in your local markets that speak your language and are capable of conducting tests and audits in state-of-the-art laboratories.
4. Work with a single-source partner who is an internationally recognised testing body with a strong presence in major markets allowing quick reaction time from anywhere in the world.
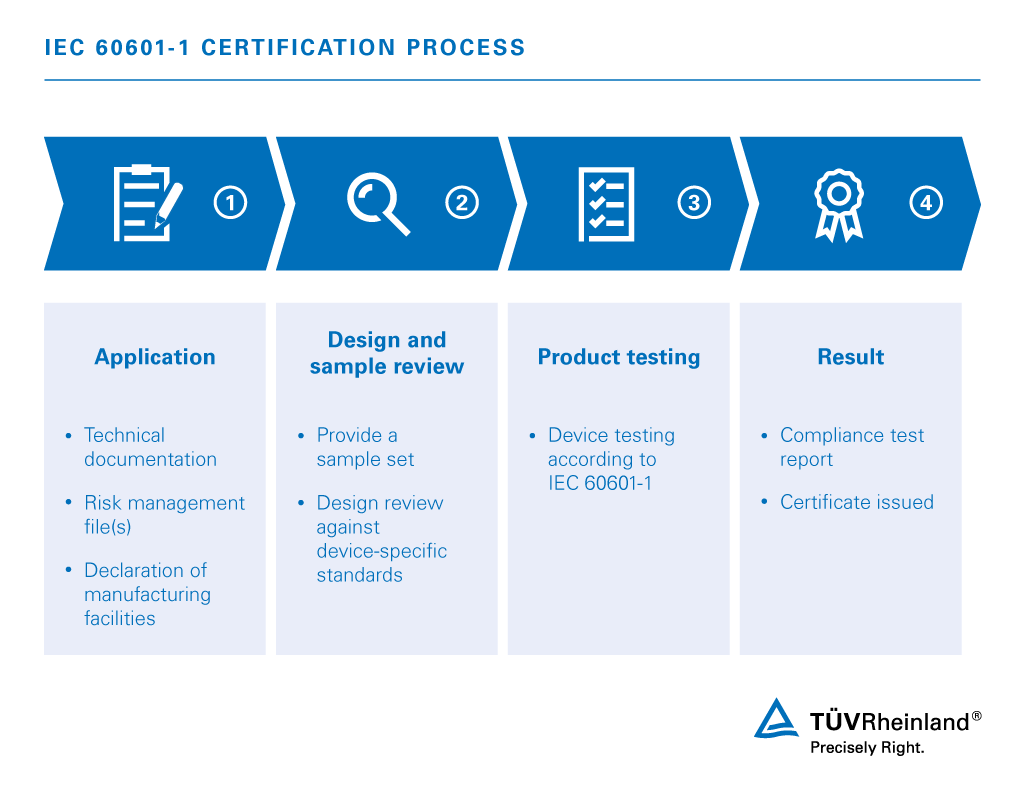
TÜV Rheinland helps you understand the often complex requirements, in addition to identifying the specific standards that apply to your product. IEC 60601 series consists of three distinct parts, each grouping of standards correlating to a specific scope.
- Base Standard: IEC 60601-1 is the base set of standards covering all general requirements for medical electrical equipment..
- Collateral Standards: IEC 60601-1-xx grouping of standards addresses horizontal issues relating to the different types of medical devices.
- Particular Standards: IEC 60601-2-xx grouping of standards depict particular requirements for specific devices.

TÜV Rheinland is one of the biggest medical scope providers worldwide. Our accredited laboratories are equipped with a wide-range of active medical device testing systems and are ready to serve you during your IEC 60601 certification. Choose a single source provider, receive all documents from one place, speak to experts around the clock, save time & money and get market-ready, fast.
We are accredited by the National Accreditation Authority, which is a full member and MRA signatory of the International Laboratory Accreditation Cooperation (ILAC). Therefore, TÜV Rheinland test reports are not only ILAC-compatible, our accreditation and laboratory testing are also of equivalent level and recognized worldwide.
Our market access services and multi-market certification programs, such as cTUVus Certification, CE Marking, INMETRO and CB scheme solutions for strategically entering and/or strengthening your position in competitive markets around the world.
Connect with us!
Back to top



