
DID YOU KNOW?
Your Medical Devices Knowledge Base
Medical devices are used for many different purposes, ranging from the treatment of small wounds to life-saving applications. On the international and national level, a complex system of rules and regulations has been established to ensure the safety of the devices.
Country-specific regulations can be difficult for manufacturers to monitor and feel confident about the conformity of their product for a target market.


TÜV Rheinland Content Library of Medical Device Services
Our experts have a deep understanding of the relevant standards, directives and regulations applicable for the medical devices business. They are also dedicated to assisting you in navigating the complexities of gaining access to your desired target markets.
We are committed to providing you with our expertise and the latest insights here. Visit this page regularly to learn the latest information on medical devices regulations, requirements and deadlines.
► "Did you know?" series on testing
In our "Did you know?" series of social media posts and related articles we will share interesting news, updates and know-how on the testing of medical devices. You can find all the published posts below, and you can also follow our LinkedIn channels (TÜV Rheinland Europe and TÜV Rheinland Medical Devices) for more information on medical device testing.
Did you know? #1
Important facts for Medical Device Manufacturers on EN 60601-1 and EN 60601-1-2. From May 27, 2024, Notified Bodies should expect compliance with the edition. 3.2 EN 60601-1 and edition 4.1 of EN 60601-1-2.
Did you know? #2
Medical device manufacturers! Did you know? You have the right to change your mind! Read our interview on the most frequently asked questions of our medical device manufacturer clients.
To read the full interview with useful questions and answers, check our article.
Did you know? #3
A revision of the standards applicable to patient monitoring equipment integrate various functionalities such as electrocardiography (ECG), invasive pressure monitoring, pulse oximetry, etc. See what changes you can prepare for.
To learn more about patient monitoring equipment, read our expert's latest article.
Stay tuned for the article →
► Other medical devices' articles
Apart from medical device testing, if you would like to learn more on medical devices' auditing and certification, please see more interesting articles from our expert colleagues below.
If you would still need more information which is not available here, please visit our website and/or get in touch with our colleagues here.
Personalized medical devices

This article allows to understand differences between custom-made medical devices belonging to group of “personalized medical devices” and other medical devices which also belong to “personalized medical devices” but are not considered as custom-made.
Alternative critical components

What happens when the critical components needed for the medical product are no longer available on the market at the end of a years-long development process? In this interview, our expert explains how manufacturers can also prepare for future challenges.
The testing of ventilators
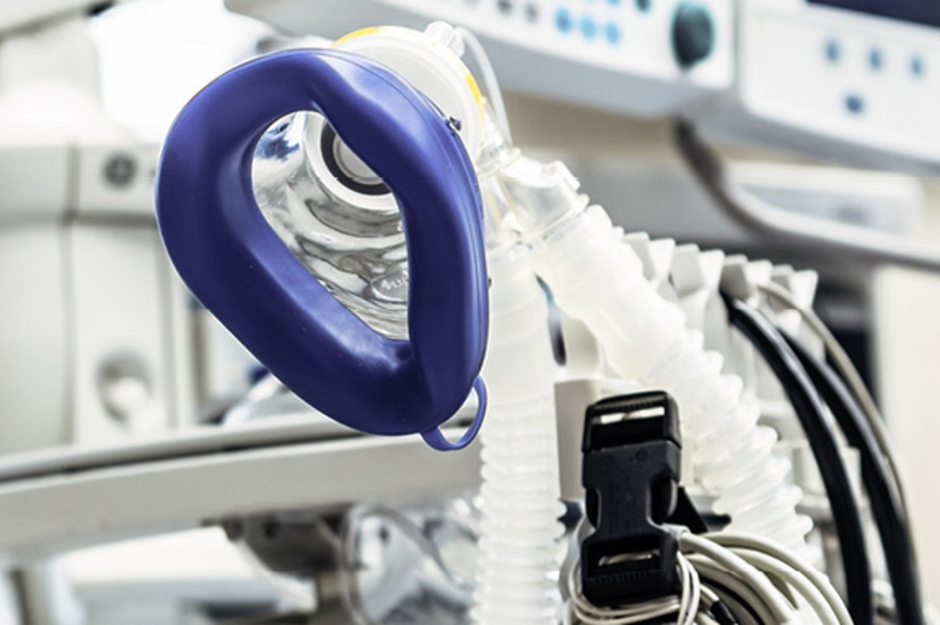
Interview with TÜV Rheinland expert Ralf Schönsteiner on ventilator testing according to EN ISO 80601-2-12:2020. Our expert Ralf Schönsteiner, head of the laboratory for active medical devices, explains what challenges manufacturers of ventilators are facing and what they should consider.
Mastering UKCA Marking

The UK government introduced the UKCA mark as clear proof that a product complies with the applicable legislation. It covers most products that previously required the CE marking. Check your comprehensive guide to UK medical devices regulations here.
Contact us!
Our experts, auditors and test laboratories are there for you – locally around the world. Please let us know how we can help!
Would you like to have more information, or do you have any specific questions?
■ Or just complete our contact form here
■ Or directly reach out to
Iwona Wrona,
Business Development Manager,
Western and Central Eastern Europe:
Looking forward to doing business with you in the near future!
Yours sincerely,
TÜV Rheinland Europe
Back to top




